Marvelous Tips About How To Write Irb Protocol
For an irb protocol submission, you will want to include specific details about the study location and context that explain risks, benefits, measures, and justifications for the use and selection of human subjects. when writing your irb protocol, you can use the tc reviewer questionsas a guide for what irb reviewers will.
How to write irb protocol. Conducting study after irb approval. If the protocol had only included parts. When completing the irb application, remember to describe the entirety of the study.
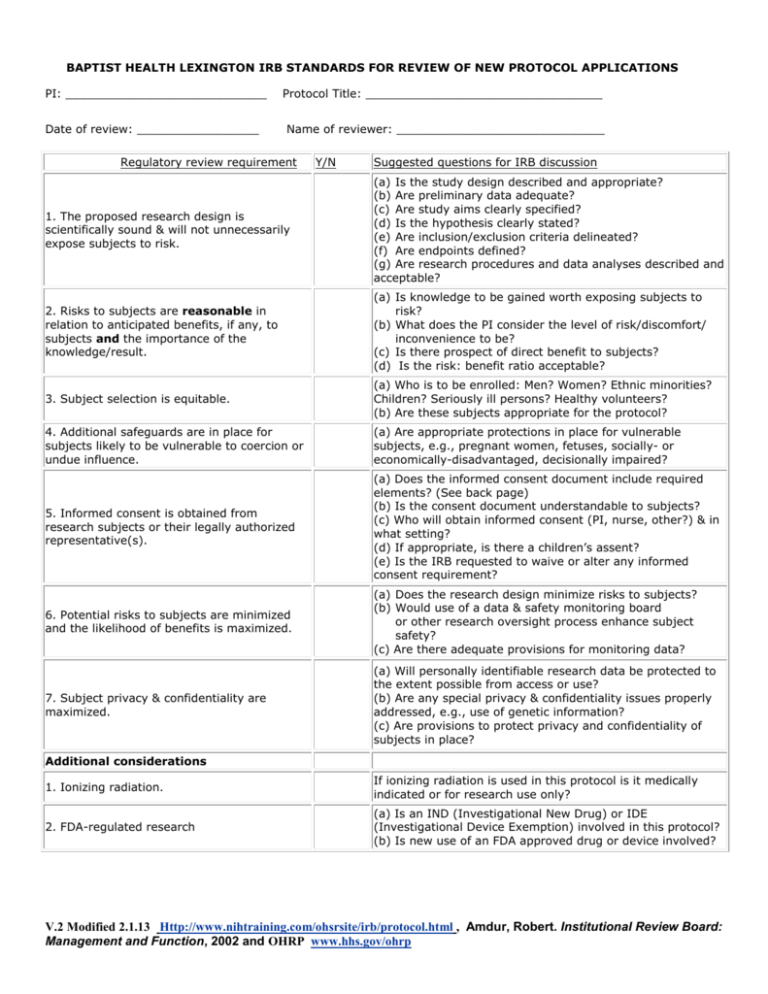
Institutional review board (irb) is a committee responsible for reviewing and approving research proposals. The irb written procedures checklist is designed to prompt a thorough evaluation of procedures essential for ensuring the protection of human research. In the get started section, complete the following:
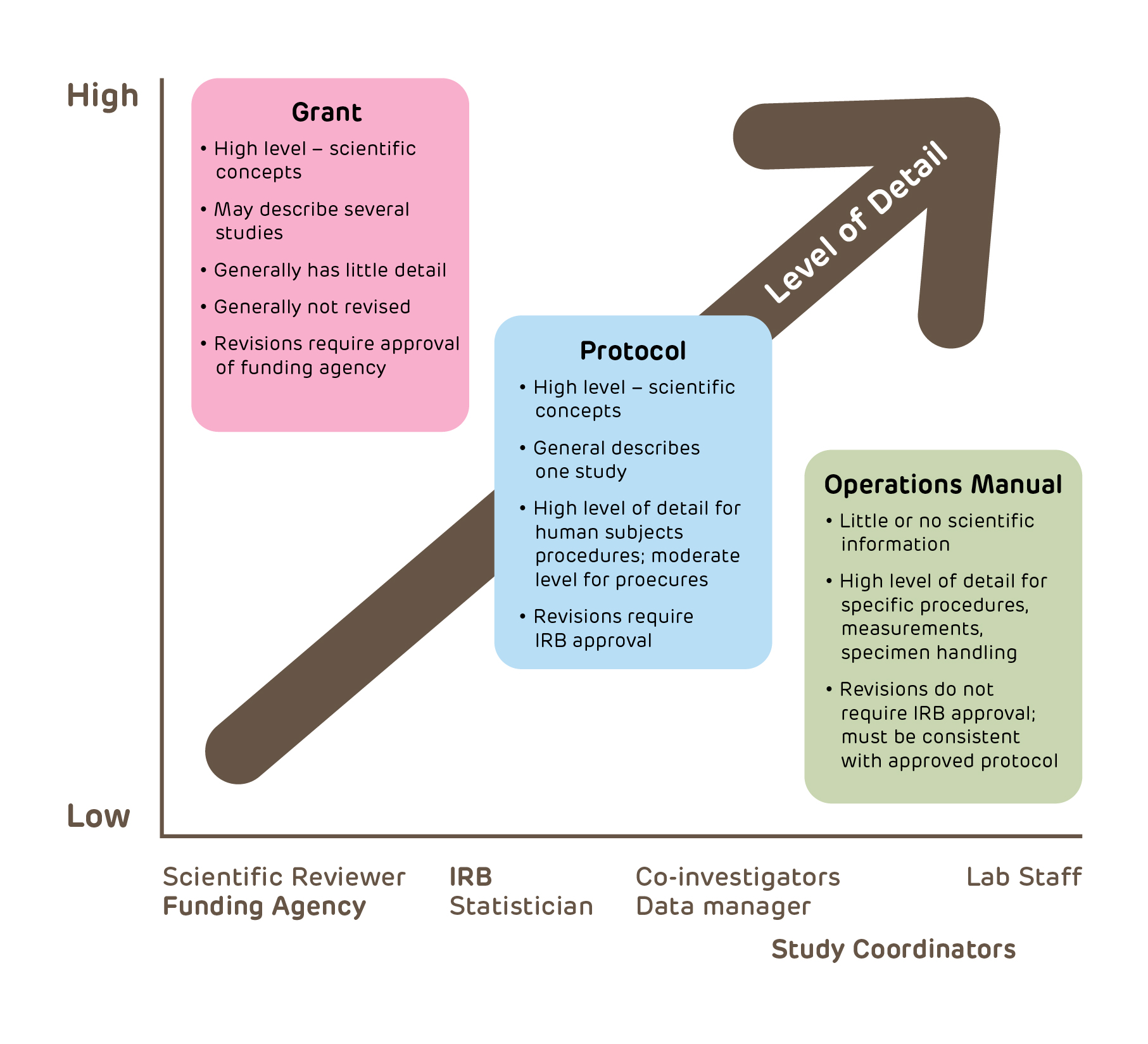
Guidance for institutions and irbs additional copies are available from:division of policy and. This part of the protocol deals specifically with existing data. How to write a human research protocol.
How to prepare an irb application. A well written protocol should address all scientific and research ethics issues. The irb office has developed protocol templates for use by the northwestern university research community to describe research/human research activities.
The irb has found that providing protocol templates and resources related to protocol writing reduces both the investigator's and the irb's work and enables. Create a new irb protocol (general) create a reliance request. Institutional review board (irb) written procedures:
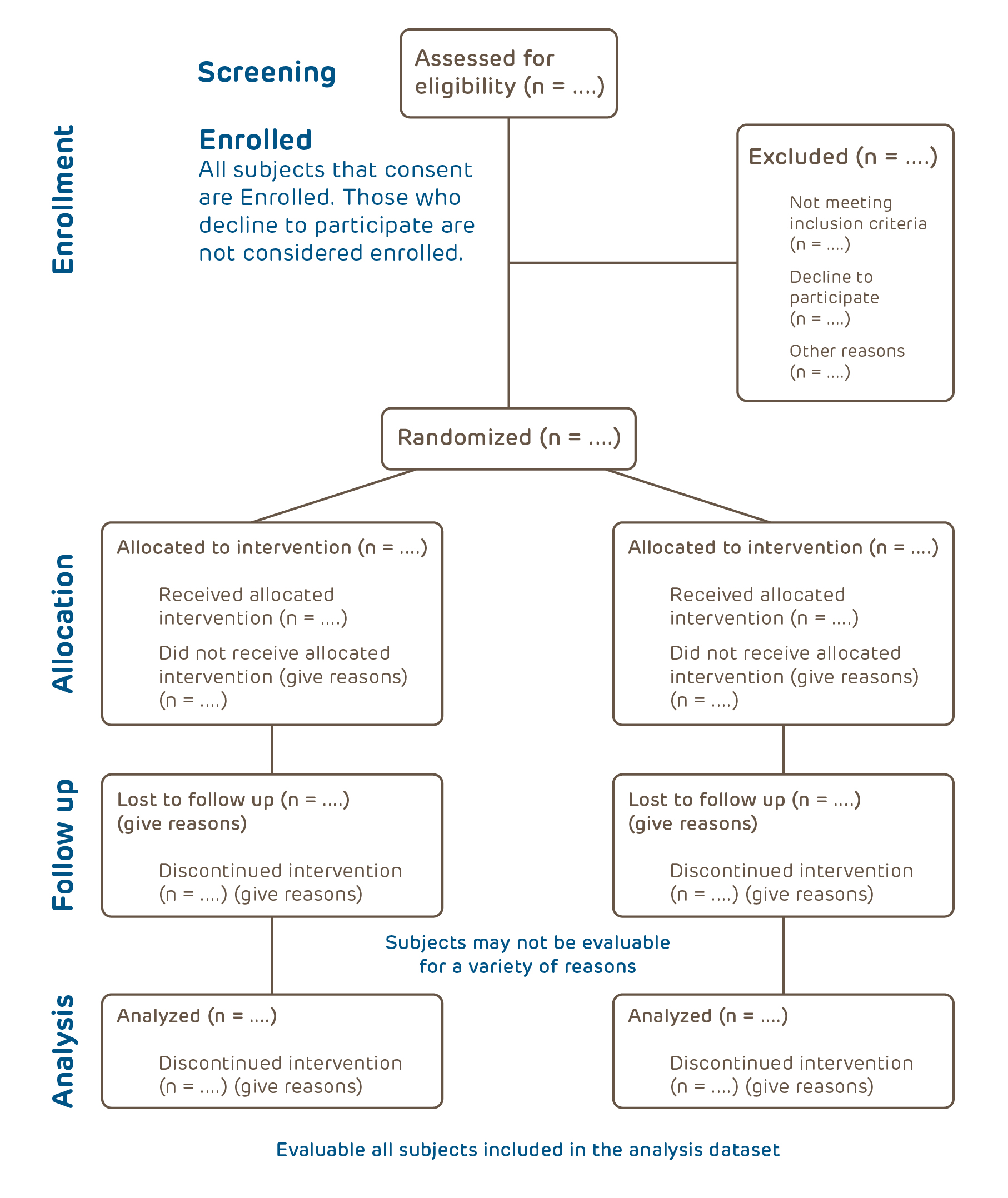
The irb application and review process is as follows (see figure 1). How to… search and report. Submitting a protocol for existing data.
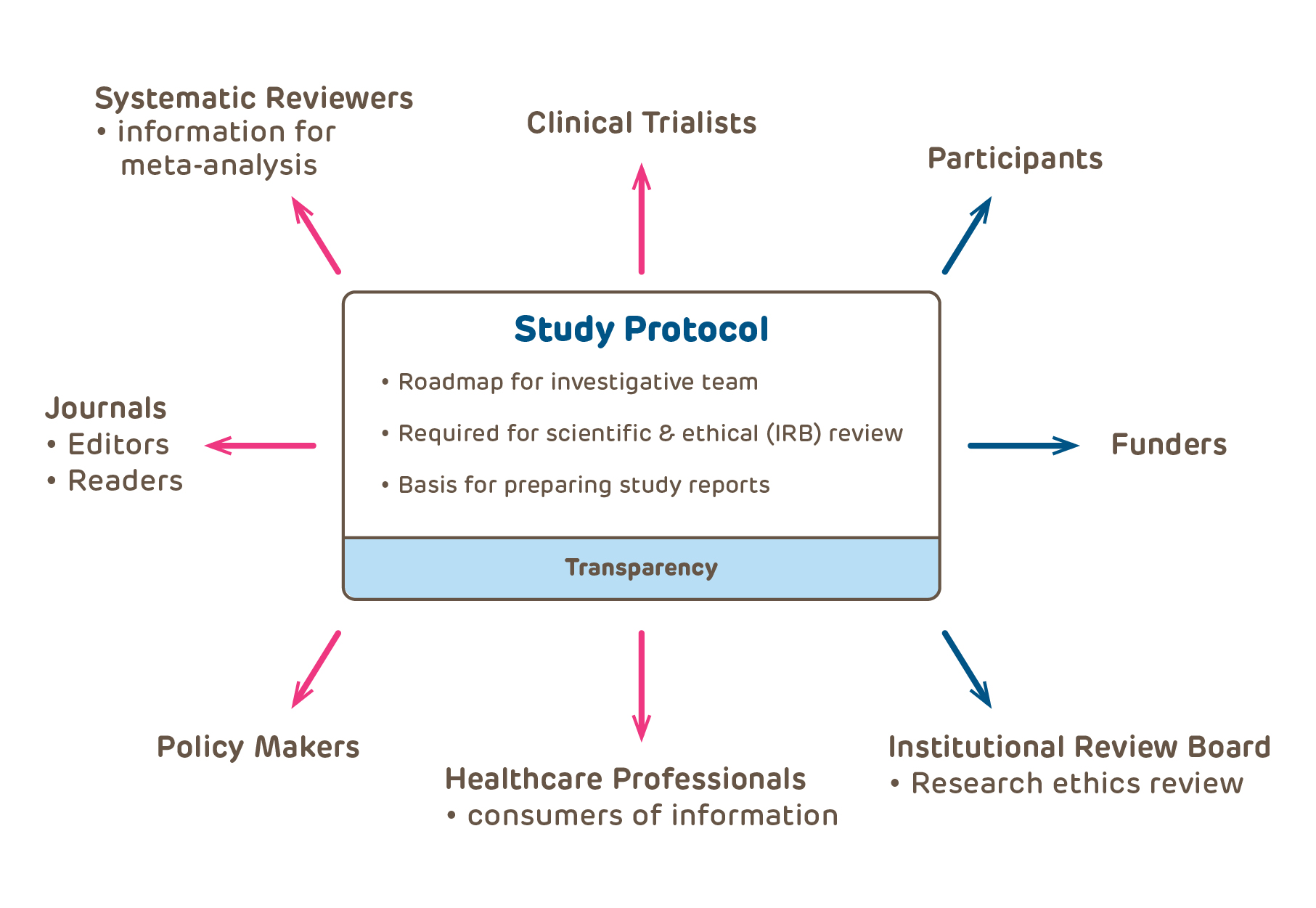
The protocol provides the irb with all of the necessary information regarding study design, conduct of the study and execution of the study. A properly written protocol will accurately describe the study design, objectives and plan, rationale for the protocol, describe study procedures to be done, describe management. Any part of your research.
What does a properly written protocol. Develop research topic with your advisor 2. If you believe your activity may not meet the definition of “human research” subject to irb oversight, contact the.
Although there are a variety of institutional. Visit tc irb’s website/how to submit/guides/writing for the irb for further tips. Imagine when completing the research protocol that you will give this to a future.
From the irb menu, select create irb protocol to start a new protocol. When writing your irb protocol, you should answer all questions on the tc irb application. These examples are applicable to the other templates.